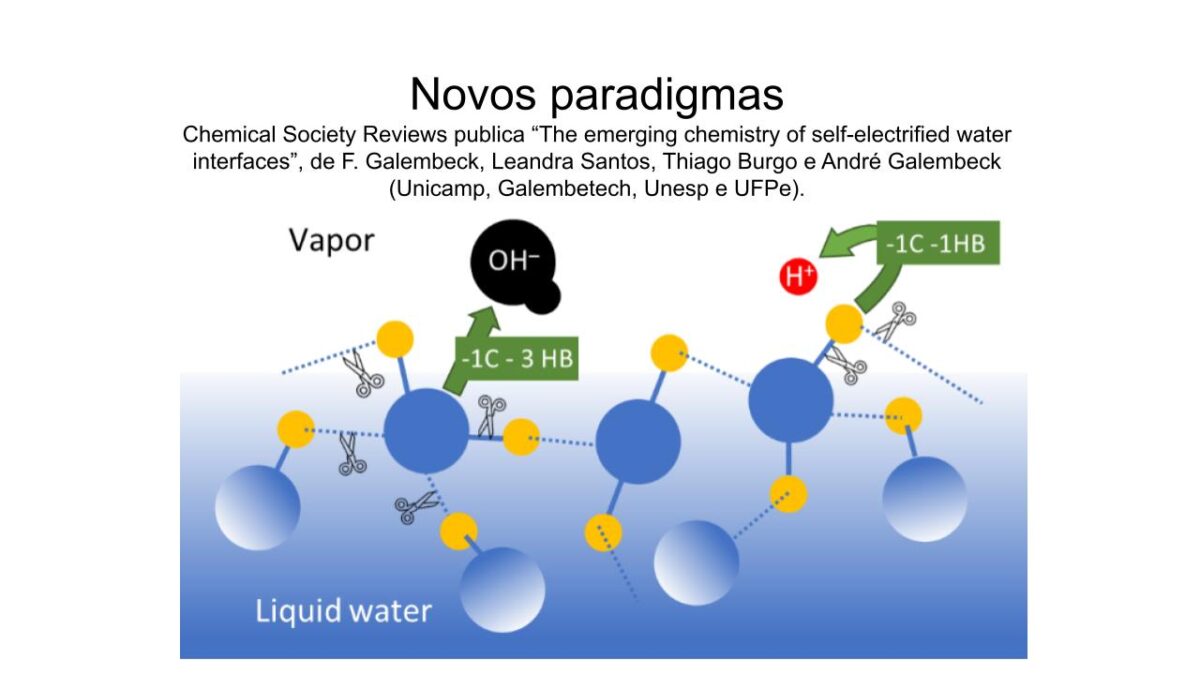
A revista Chemical Society Reviews publicou o artigo “The emerging chemistry of self-electrified water interfaces” de Fernando Galembeck, Leandra P. Santos, Thiago A. L. Burgo e Andre Galembeck, DOI: 10.1039/d3cs00763d, em Open Access. Os quatro autores são pesquisadores participantes do Inomat, atuando em quatro instituições participantes.
Esse artigo demonstra que a matéria macroscópica é sempre eletrizada, devido à onipresente eletrização de interfaces aquosas e ampla distribuição da água, na superfície da Terra. Eletrização de interfaces causa mudanças profundas na reatividade química, em mudanças de fase e morfologia de cristais, viabilizando novos processos de produção de energia, de hidrogênio e outras substâncias importantes. O resumo do artigo é o seguinte:
“Water is known for dissipating electrostatic charges, but it is also a universal agent of matter electrification, creating charged domains in any material contacting or containing it. This new role of water was discovered during the current century. It is proven in a fast-growing number of publications reporting direct experimental measurements of excess charge and electric potential. It is indirectly verified by its success in explaining surprising phenomena in chemical synthesis, electric power generation, metastability, and phase transition kinetics. Additionally, electrification by water is opening the way for developing green technologies that are fully compatible with the environment and have great potential to contribute to sustainability. Electrification by water shows that polyphasic matter is a charge mosaic, converging with the Maxwell–Wagner–Sillars effect, which was discovered one century ago but is still often ignored. Electrified sites in a real system are niches showing various local electrochemical potentials for the charged species. Thus, the electrified mosaics display variable chemical reactivity and mass transfer patterns. Water contributes to interfacial electrification from its singular structural, electric, mixing, adsorption, and absorption properties. A long list of previously unexpected consequences of interfacial electrification includes: “on-water” reactions of chemicals dispersed in water that defy current chemical wisdom; reactions in electrified water microdroplets that do not occur in bulk water, transforming the droplets in microreactors; and lowered surface tension of water, modifying wetting, spreading, adhesion, cohesion, and other properties of matter. Asymmetric capacitors charged by moisture and water are now promising alternative equipment for simultaneously producing electric power and green hydrogen, requiring only ambient thermal energy. Changing surface tension by interfacial electrification also modifies phase-change kinetics, eliminating metastability that is the root of catastrophic electric discharges and destructive explosions. It also changes crystal habits, producing needles and dendrites that shorten battery life. These recent findings derive from a single factor, water’s ability to electrify matter, touching on the most relevant aspects of chemistry. They create tremendous scientific opportunities to understand the matter better, and a new chemistry based on electrified interfaces is now emerging.”